Alzheimer’s disease and other dementias grew in incidence and prevalence by 147.95% and 160.84%, respectively, from 1990 to 2019. This indicates how widespread Alzheimer’s has become, complicated by the fact that there is no cure.
Patients and caregivers have been searching for therapies for a long time, and each new study brings them newfound hope.
Eisai, a Japanese pharmaceutical company, discovered one particular drug, a new treatment for Alzheimer, that improved cognitive function in Alzheimer’s patients, as published in the New England Journal of Medicine.
This Mind The Graph article will walk you through this innovative treatment and explain how it can improve the trajectory of Alzheimer’s patients.
All you need to know about the new treatment for Alzheimer
This new treatment for Alzheimer’s comes from a medication named Lecanemab, discovered during an 18-month phase 3 trial including people aged 50 to 90 with early Alzheimer’s disease. To be eligible for the experiment, these individuals must have moderate cognitive impairment or mild dementia caused by Alzheimer’s disease, as well as signs of amyloid plaques in their brains.
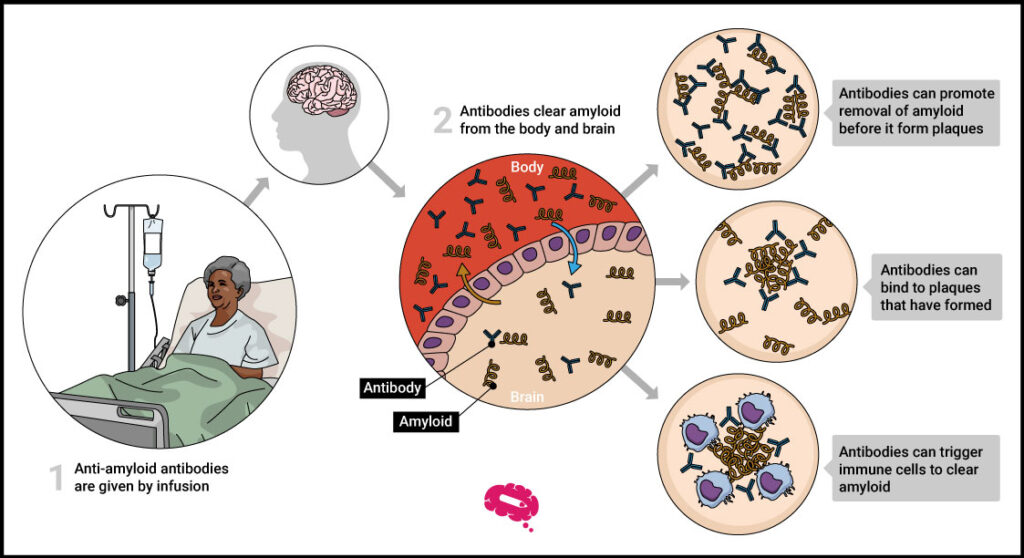
Patients in this experiment would receive either intravenous Lecanemab or a placebo. The alterations were measured after 18 months. After this period, patients with early-stage Alzheimer’s who took Lecanemab experienced 27% less cognitive impairment than those who received a placebo. This corresponds to 0.45 points on an 18-point CDR-SB.
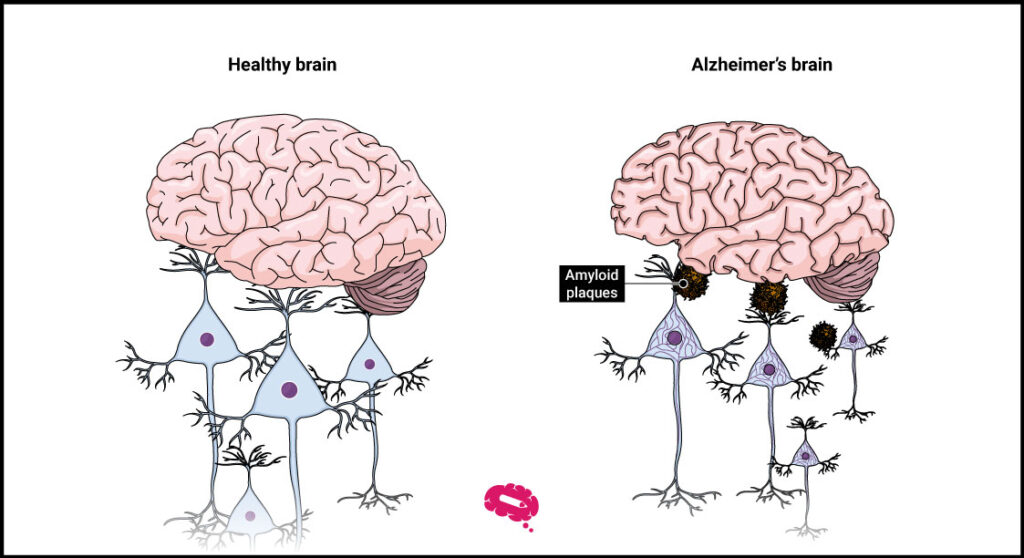
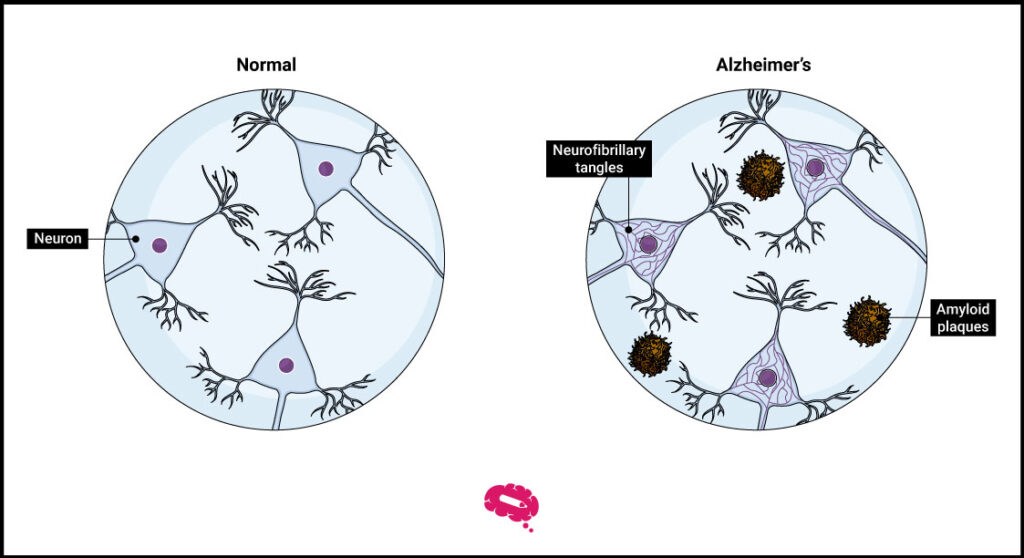
The primary goal for Alzheimer’s researchers was to identify a successful treatment to combat the disease’s key point: amyloid plaques in the brain, a protein that disrupts neurons and other cells. This protein is produced through the breakdown of a bigger protein, termed amyloid precursor protein.
So far, finding a new treatment for Alzheimer’s and a medication capable of blocking amyloid plaques while simultaneously alleviating symptoms has been almost a lost war. Nevertheless, Lecanemab was the first drug to do so.
Many more studies comparable to this one have been carried out in the past with no similar findings. For example, Aducanumab, also known as Aduhelm, is a drug that became available in 2021. Similarly to Lecanemab, those receiving Aducanumab improved in cognitive tests compared to those taking a placebo. The effects of Aducanumab were revealed in an earlier phase 1 research that included a smaller number of patients and was aimed at examining the therapy’s safety rather than its efficacy.
Because the advantages of the medicine were not reliably and consistently replicated in phase 3 studies, the Centers for Medicare and Medicaid Services chose not to pay the high cost of the drug, which costs $56,000 per year and requires more trials to establish the treatment’s effectiveness.
Although both drugs appear to be relatively similar, it is essential to highlight how differently they act, which is why scientists believe Lecanemab will have a far greater effect in modifying the course of Alzheimer’s. Aducanumab interacts with amyloid proteins that have already been grouped together. Lecanemab, on the other hand, intervenes sooner, targeting “protofibrils,” or filaments that will eventually become plaques.
Are there any side effects?
Lecanemab appears to have fewer adverse effects than Aducanumab up to this point. Eisai, the company that performed the trial, claimed that 12.6% of patients suffered ARIA-E brain inflammation, which can be deadly but is curable if the medicine is stopped or the dose is lowered.
On the other hand, approximately one-third of those on Aducanumab developed ARIA-E during the drug’s phase 3 trial.
During the Lecanemab experiment, 13 patients died, and they were nearly evenly split across the treatment and placebo groups, with seven fatalities in the treatment group and six in the placebo group.
The FDA will review everything that occurred throughout the experiment, including the 13 deaths, before actually releasing the drug. However, the drug appears to be promising so far.
Turn your data into easy-to-understand
According to a study, human brains are built to process visuals, and they can do it at breakneck speed. Images can be digested at least twice as quickly as text can be. So, use this information to your advantage and select a tool, like Mind The Graph, to easily create aesthetically attractive materials for your work, which can transform large amounts of text into spectacular and easy-to-see visuals.

Subscribe to our newsletter
Exclusive high quality content about effective visual
communication in science.