Ionic liquids are fascinating compounds that have gained significant attention among industrial and academic researchers in recent years. Unlike traditional liquids composed of molecules, ionic liquids are made up of ions, which are charged particles. This unique composition gives them several intriguing properties, such as low volatility, high thermal stability, and excellent conductivity. These characteristics make them suitable for a wide range of applications, including energy storage, catalysis, and electrochemistry. In this guide, we will explore the fundamentals of ionic liquids, their synthesis, characterization, and various practical applications. Whether you are a seasoned scientist or a curious beginner, this comprehensive guide will provide you with the necessary knowledge to understand and utilize these remarkable substances in your research.
What Are Ionic Liquids?
Ionic liquids are a unique class of organic compounds that are entirely composed of ions. Unlike typical liquids, which consist of neutral molecules, ionic liquids are entirely made up of positive (cations) and negative (anions) ions. These ions are held together by electrostatic forces, much like the forces that keep table salt (a solid ionic compound) together.
However, what sets ionic liquids (IL) apart is the unique combination of ions which results often in a liquid state at room temperature. This is contrary to our expectations, as ionic compounds are usually solids at room temperature. Indeed, by definition, ILs are defined as organic salts with melting temperatures below 100 °C. The reason behind this is the asymmetrical and bulky nature of the ions used in ionic liquids, which disrupts the formation of a regular, solid crystalline structure.
These properties make ionic liquids an intriguing area of study for scientists and researchers across various fields. Understanding what ionic liquids are is the first step towards unlocking their potential in scientific research and applications.
When Were Ionic Liquids Discovered?
The first IL was discovered by Paul Walden in 1914. However, it took nearly a century for ILs to become a relevant scientific topic. Fast forward to 1934, a pivotal moment when a patent was filed for the use of quaternary ammonium salts in liquid form, turning ILs into an industrial game-changer.
How Are Ionic Liquids Synthesized?
Generally, the synthesis of ILs occurs through the following steps:
- IL Design: select a cation and an anion based on the desired properties of the ionic liquid. Common cations include imidazolium, pyridinium, and ammonium, while common anions include chloride, bromide, and acetate. The formation of the cation can be performed by a protonation or alkylation reaction.
- Ionic Exchange: mix the selected cation and anion precursors in a suitable solvent or under specific conditions to facilitate the ionic exchange reaction. The reaction often involves heating and stirring.
- Purification: after the reaction, purify the resulting ionic liquid to remove any unreacted precursors, by-products, or impurities. Common purification methods include precipitation, filtration, distillation, or liquid-liquid extraction.
- Characterization: analyze the synthesized ionic liquid to confirm its identity and purity. Techniques such as nuclear magnetic resonance (NMR), infrared spectroscopy (IR), and mass spectrometry (MS) can be used for characterization.
Why Are Ionic Liquids So Special?
The ability to dissolve a variety of organic substances and contribute to liquid-liquid extraction, making them a top choice for processing poorly soluble biopolymers. But what truly sets ILs apart is their versatility. This arises from the extensive options for both cations and anions. By combining various cations and anions a collection of approximately 1018 distinct ILs can be created. This diversity translates into a host of features – high chemical stability, non-flammability, high ionic conductivity, low vapor pressure, and thermal stability – making ILs the go-to solution for a multitude of applications.
Exploring Ionic Liquids
Considering all the unique properties of ILs, they present as promising compounds for different applications, such as:
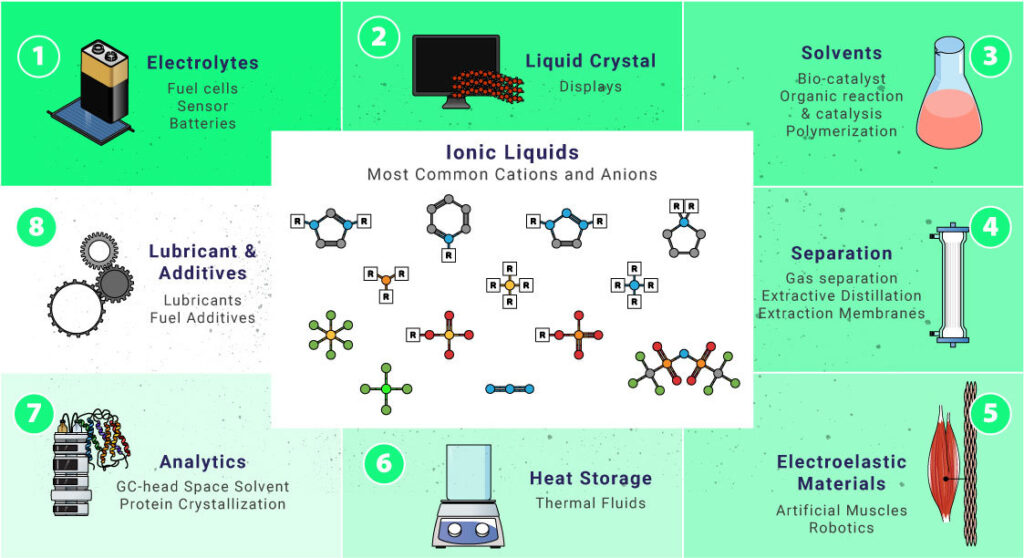
Electrolytes
An electrolyte is a substance with ions that allows electrical conduction through the movement of ions, without conducting electrons. In the realm of energy storage and battery technology, ILs serve as exceptional electrolytes. Their unique ionic nature and stability make them promising candidates for enhancing the performance and efficiency of batteries, contributing to the advancement of renewable energy solutions.
Liquid Crystals
Liquid crystal (LC) represents an intermediary phase that exhibits properties of both crystals and liquids. In this state, a liquid crystal can flow similarly to a liquid, yet its molecules maintain a specific crystal-like orientation. The key characteristic for liquid crystalline behavior appears to be a long, rigid, and highly anisotropic structure. Many materials demonstrating liquid crystalline properties are constructed based on aromatic rings to achieve this desired structural arrangement.
ILs play a pivotal role in the development of liquid crystal technology, a field crucial for electronic displays and devices (e.g. calculators, digital watches, oscillographic systems, television displays). Their ability to exhibit liquid crystalline phases offers a platform for designing advanced materials with tailored properties, influencing the landscape of modern electronics.
Solvents
A solvent is a substance that dissolves another substance (solute) to form a homogeneous mixture (solution). As solvents, ILs showcase their versatility by dissolving a wide range of substances. This property makes them valuable tools in various chemical processes, offering a cleaner and more efficient alternative to traditional solvents, especially in green chemistry initiatives. The IL solvents are mainly composed of salts derived from 1-methylimidazole, specifically 1-alkyl-3-methylimidazolium. Examples include 1-ethyl-3-methyl (EMIM), 1-butyl-3-methyl (BMIM), 1-octyl-3-methyl (OMIM), 1-decyl-3-methyl (DMIM), and 1-dodecyl-3-methyl (dodecylMIM). One of the main advantages of ILs is the possibility of recovering these compounds at the end of the process.
Lubricants And Additives
Additives and lubricants include substances enhancing base oil characteristics (viscosity index modifiers), protective elements (antioxidants), and compounds providing new properties while safeguarding engine metal surfaces. ILs demonstrate efficacy as lubricants and additives, contributing to improved mechanical system performance. Their unique lubricating properties reduce friction and wear, enhancing the overall efficiency and longevity of machinery in diverse industrial applications.
Separation Techniques
Ionic Liquids (ILs) find utility across diverse separation applications, including their use in ionic liquid-supported membranes, as additives in mobile phases, surface-bonded stationary phases in chromatography separations, and as extraction solvents in sample preparations. Their adaptability stems from the ability to be formulated with different cations and anions, thereby altering the properties and phase behavior of liquids.
Heat Storage
Due to their exceptional thermal stability, low vapor pressure, non-flammability, and wide temperature range in the liquid state, ionic liquids (ILs) find applications in heat transfer and thermal storage. In the quest for sustainable energy solutions, ILs excel in heat storage, efficiently absorbing and releasing heat. This positions them as promising materials for thermal energy storage and transference, contributing to the development of sustainable and eco-friendly industrial processes.
Electroactive Materials
ILs exhibit electroelastic properties that hold promise for the development of electroactive materials and devices. These materials can be tuned and controlled in response to electric fields, opening avenues for advancements in electronics, sensors, and actuators.
In essence, the versatility of ILs spans across a spectrum of scientific and industrial applications, making them indispensable contributors to advancements in energy, materials, and chemical processes. The ongoing research and exploration in these diverse fields continue to unveil new possibilities for Ionic Liquids, solidifying their role as transformative agents in contemporary science and technology.
Ionic Liquids are versatile substances. Check some of the most common cations and anions that can form ILs and their potential applications in daily life.
New Challenges On Ionic Liquids
As the field of Ionic Liquids (ILs) continues to evolve, several new challenges have emerged. One key challenge lies in the development of more sustainable and environmentally friendly ILs, addressing concerns related to toxicity, bioavailability and biodegradability. Additionally, there is a growing need for a deeper understanding of the long-term effects and potential ecological impact of ILs, especially as their usage expands across various industries. Another significant challenge involves optimizing the synthesis and production processes to enhance cost-effectiveness and scalability, making ILs more feasible for widespread industrial applications. Furthermore, researchers are actively exploring methods to improve the recyclability and reusability of ILs, aiming to minimize waste and environmental impact. As ILs find applications in increasingly complex systems, such as biological and pharmaceutical processes, there is a demand for tailored ILs with enhanced specificity and selectivity. Overcoming these challenges will not only contribute to the maturation of the field but also pave the way for the responsible and sustainable utilization of Ionic Liquids in diverse applications.
Creating Effective Visualizations Of Ionic Liquids With Mind the Graph
Visualizing the Structure of Ionic Liquids
Visualizing the structure of ionic liquids, which consist entirely of ions, is a challenge. Mind the Graph, an online platform for scientific figures provides a valuable tool for this. With scientifically accurate illustrations, it helps represent the arrangement and interactions of cations and anions within ionic liquids. This facilitates the creation of clear and visually appealing depictions, enhancing the understanding and communication of these compounds’ unique properties, synthesis processes, and interactions with other compounds in both research and scientific discourse
Visually Appealing Figures For Your Research
Mind the Graph: Transforming scientific figures with ease and impact. Create visually stunning figures that captivate audiences, communicate complex concepts, and elevate the impact of your research. Experience the power of visual communication with Mind the Graph and revolutionize your scientific presentations. Sign up for free!

Subscribe to our newsletter
Exclusive high quality content about effective visual
communication in science.