Carbon dioxide is a common gas present in the atmosphere. Normally, the Earth’s carbon cycle maintains a natural balance of carbon in the atmosphere, land, and ocean through the “breathing of the planet”. However, human activities as emission of fossil fuels are breaking the balance of carbon cycle causing climate change, as increasing the greenhouse effect and ocean acidification.
So, to understand the consequences of ocean acidification, you need first to recap the carbon cycle. I wrote about that here1.
The greenhouse effect allows the life on Earth, by trapping the heat in the atmosphere, warming the planet. I showed how it works here1. However, despite the importance of it, the increasing of carbon dioxide has turned this into a problem.
But not all of the excess carbon dioxide stays in the atmosphere. Scientists estimate that the oceans absorbed one-third of all the carbon dioxide produced by human activities. The ocean’s removal of carbon dioxide from the atmosphere helps delay the extent of climate change. However, this benefit has come at a cost.
Carbon dioxide and the chemistry of the ocean
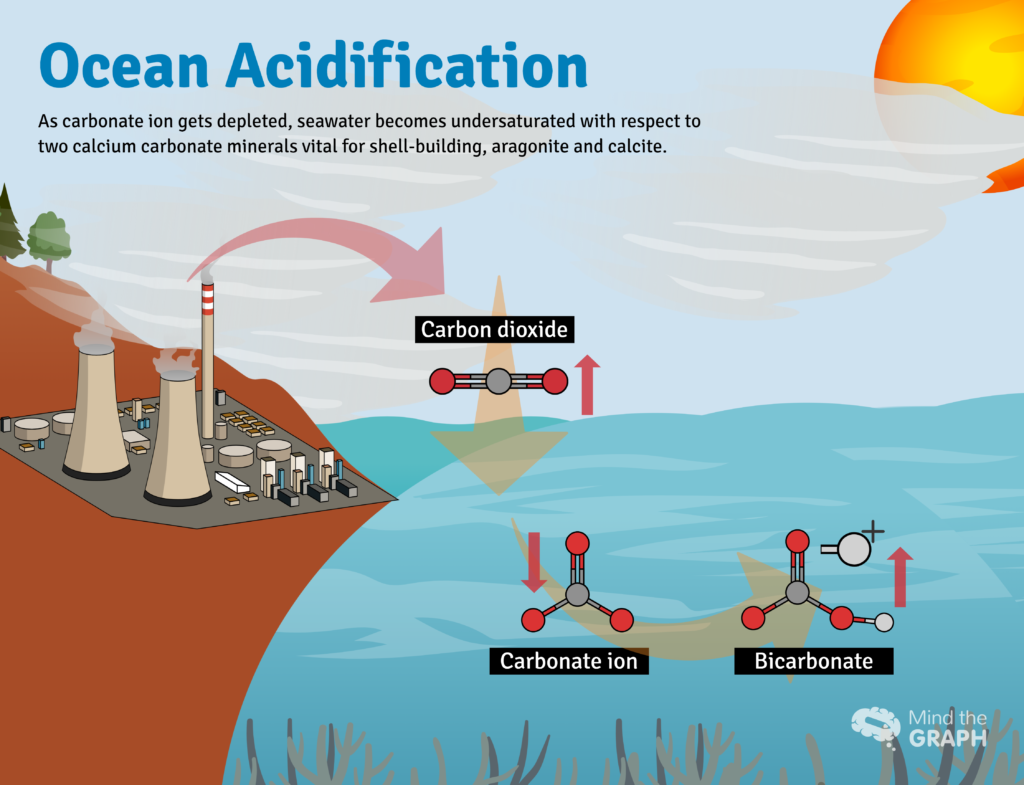
Once dissolved in seawater, CO2 reacts with water (H2O) to form carbonic acid: H2CO3: CO2 + H2O ↔ H2CO3. Carbonic acid dissolves rapidly to form H+ ions (an acid) and bicarbonate, HCO3- (a base). Seawater is naturally saturated with another base, carbonate ion (CO3−2) that acts like an antacid to neutralize the H+, forming more bicarbonate. The net reaction looks like this: CO2 + H2O + CO3−2→ 2HCO3-
The absorption of carbon dioxide is fundamentally changing the chemistry of the ocean by triggering reactions that make seawater more acidic, a phenomenon called ocean acidification. In fact, ocean has become nearly 30 percent more acidic than it was at the beginning of the Industrial Era. It is a change larger and faster than seen in the fossil record going back at least 800,000 years, before the appearance of vertebrates and plants in the fossil record.
How will ocean acidification impact marine life such as fish, corals, and shellfish?
As the concentration of hydrogen ions increases, the water becomes more acidic. Besides that, the carbonate ions become less abundant.
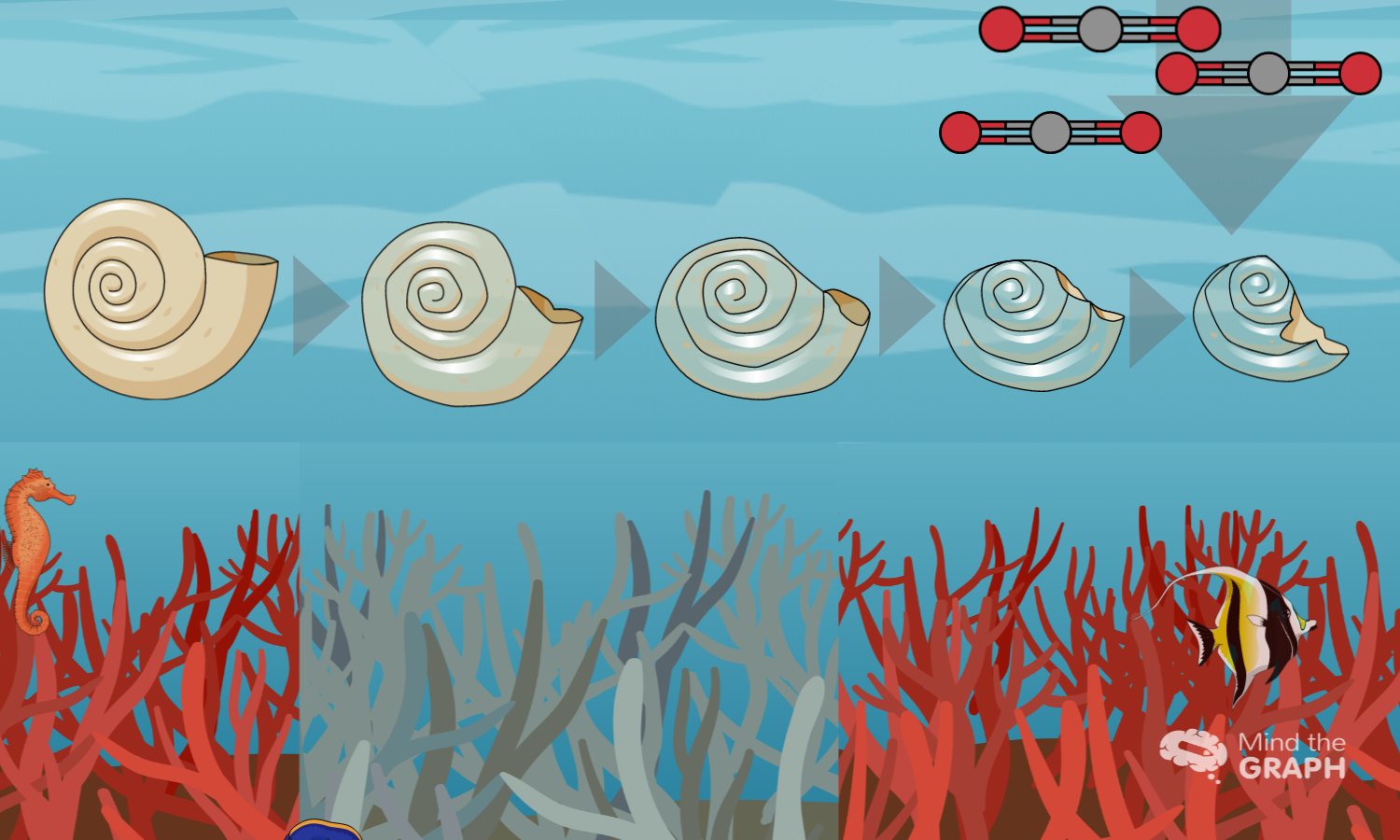
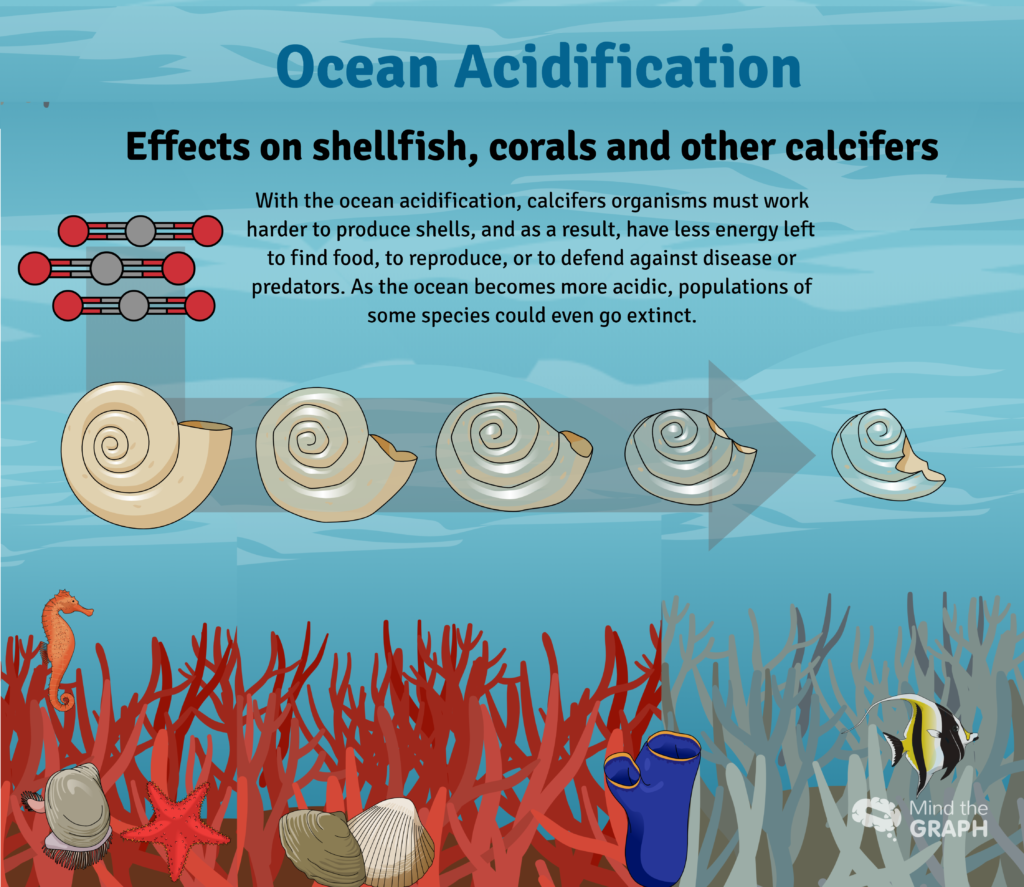
Some of the extra hydrogen ions react with carbonate ions to form more bicarbonate. As carbonate becomes less abundant, these organisms, such as corals and clams, have more difficulty building and maintaining their shells and skeletons. Increased acidity can even cause some carbonate shells and skeletons to dissolve. Hydrogen ions react with the solid calcium carbonate and convert it to soluble bicarbonate and calcium ions.
Amongst the mixture of tiny plants and animals that make up plankton lives a tiny sea snail called the pteropod. Despite their small size, pteropods are an important source of food for many species, including fish, seals, and whales. But pteropods have delicate calcium carbonate shells that are vulnerable to ocean acidification. In a series of experiments, pteropod shells were placed in seawater at the pH (acidity) projected for the Southern Ocean by 2100. Within 48 hours, the pteropod shells began to dissolve.
Visualizing your research
Visual resources like infographics and videos are a powerful way to communicate science. I created all these infographics using Mind the graph, an online platform that allows scientists to create eye-catching materials.

Subscribe to our newsletter
Exclusive high quality content about effective visual
communication in science.